What Is a Euploid Embryo?
Medically reviewed by Linda Streety, RN, BSN
You’ve heard that higher quality embryos are more likely to be “euploid”, but what does this mean, and why does it matter? Below, we’ll talk about euploid, aneuploid, and mosaic embryos, and the different tests your fertility clinic can conduct on your embryos to maximize your chance of success with in vitro fertilization (IVF).
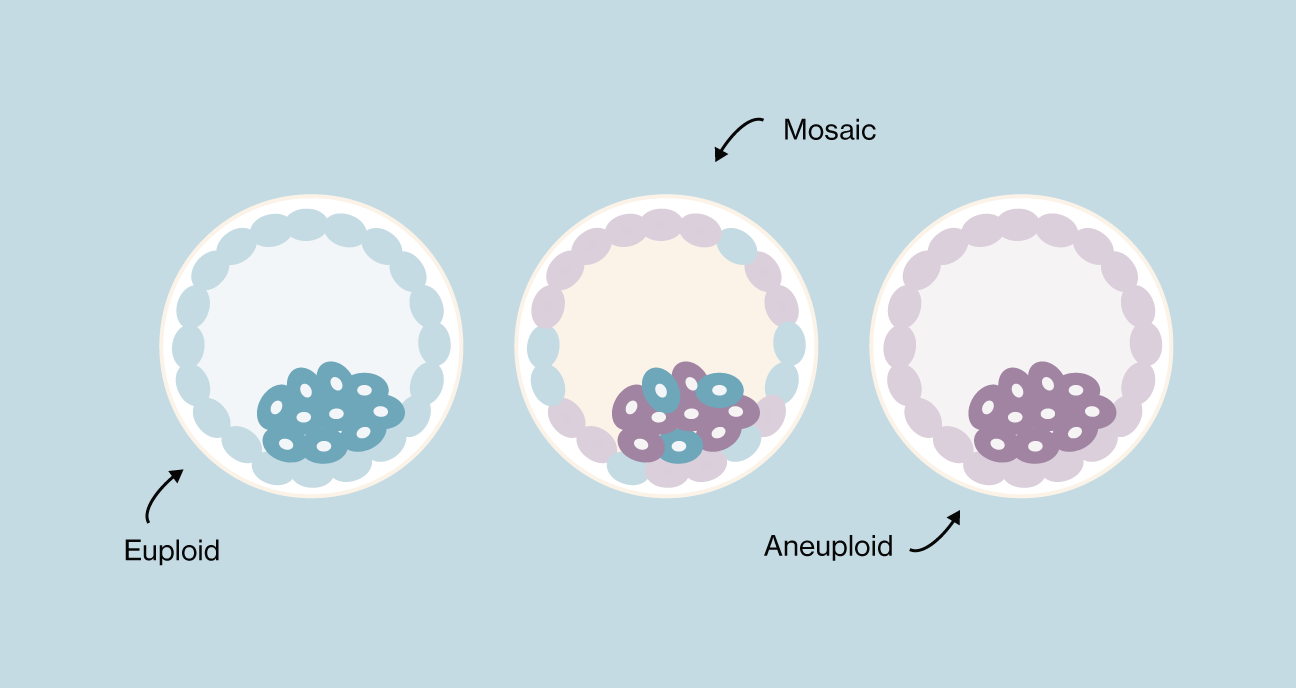
Euploid vs. Aneuploid vs. Mosaic
Euploid embryo
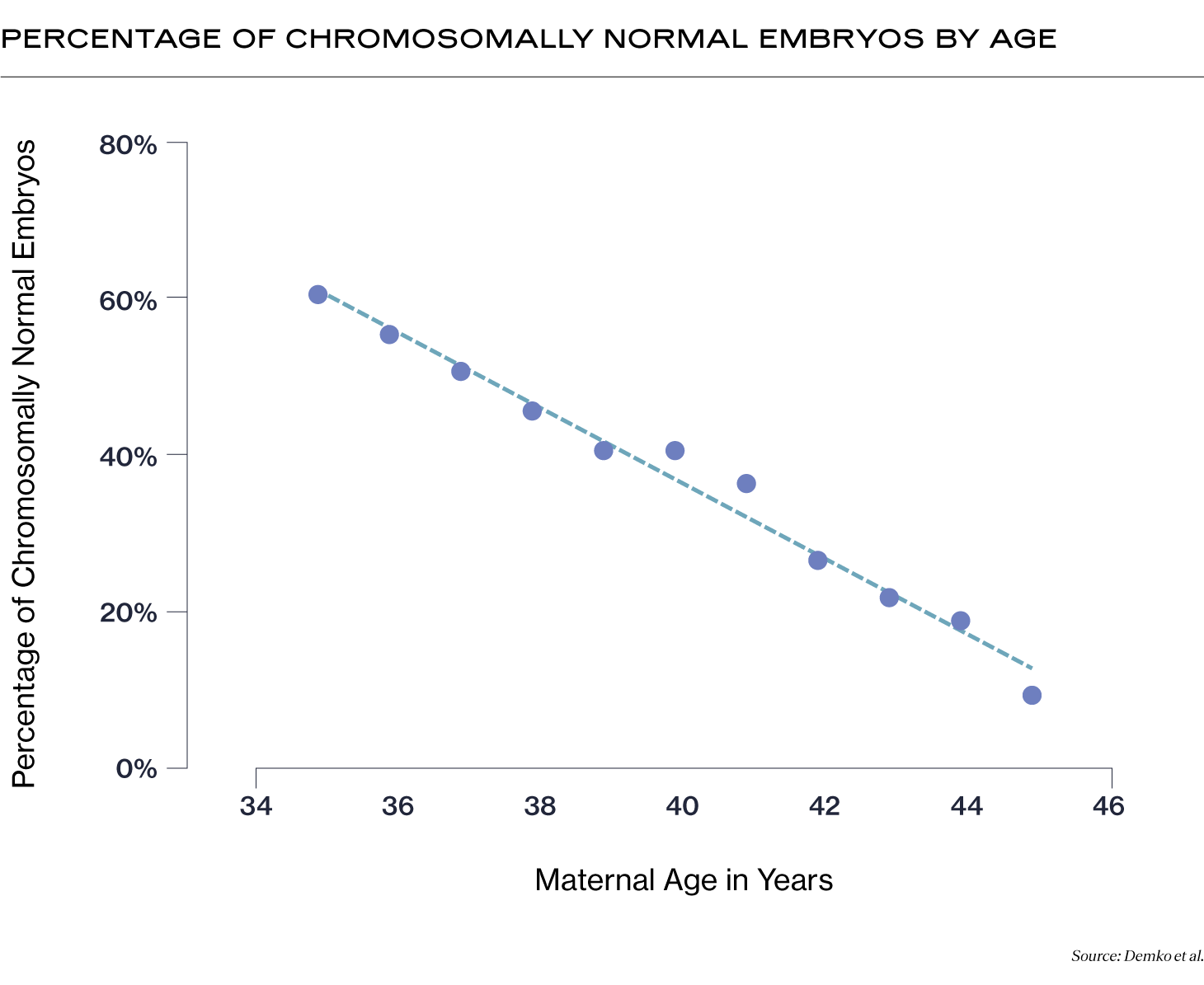
Also known as a “normal” embryo, a euploid embryo has 46 chromosomes. Euploid embryos are more likely to implant, less likely to result in miscarriage, and less likely to result in a baby with intellectual or physical challenges caused by chromosomal abnormalities. The percentage of euploid embryos you may have decreases with your age at the time of egg retrieval (1).
Aneuploid embryo
Also known as an “abnormal” embryo, an aneuploid embryo has the incorrect number of chromosomes. Rather than 46 chromosomes, an aneuploid embryo has one or more missing chromosomes (45 or fewer), or extra chromosomes (47 or more). Aneuploid embryos are significantly more likely to result in implantation failure or pregnancy loss (2). If an aneuploid embryo transfer leads to a live birth, the baby may have intellectual or physical challenges due to chromosomal abnormalities. Some chromosomal disorders that result from aneuploid embryos include Down syndrome, Turner syndrome, and Patau syndrome, among others.
Mosaic embryo
A mosaic embryo has a mix of cells with normal chromosomes and cells with abnormal chromosomes.
Aneuploid and mosaic embryos do not automatically mean that a pregnancy with one of them will not result in a healthy baby. While the chance of implantation is lower and miscarriage is higher, embryos with chromosomal abnormalities can, in some cases, self-correct and develop into a baby with normal chromosomes (3). This is particularly the case with mosaic embryos (4), and especially low to medium-grade mosaic embryos (in which fewer than 50% of the cells are impacted by mosaicism) (5).
Because of this, while your clinician may favor euploid embryos for transfer, they may not rule out a mosaic embryo. Success rates for implantation and live birth are lower for mosaic embryos than euploid embryos, but they may enable some patients (especially low-prognosis patients) to have a healthy pregnancy and baby!
| Implantation Rate | Miscarriage Rate | Pregnancy & Birth Rate | |
|---|---|---|---|
| Euploid Embryo | 57.2% | 8.6% | 52.3% |
| Mosaic Embryo | 46.5% | 20.4% | 37% |
| Euploid Embryo | |
|---|---|
| Implantation Rate | 57.2% |
| Miscarriage Rate | 8.6% |
| Pregnancy & Birth Rate | 52.3% |
| Mosaic Embryo | |
| Implantation Rate | 46.5% |
| Miscarriage Rate | 20.4% |
| Overall Pregnancy / Birth Rate | 37% |
How can you tell if an embryo is euploid?
Embryologists have two primary methods for assessing embryos to ensure that the embryos they transfer have the best chance of success: embryo grading and preimplantation genetic testing (PGT).
Embryo Grading
After an embryo has grown for 5-6 days in the lab, the embryologist will look at it under a high power microscope and assess embryo quality based on three quality scores: expansion (how expanded the embryo cavity is), the inner cell mass (how tightly packed the cells are in the center of the embryo), and the trophectoderm (the cells forming the outer layer of the embryo). Embryos with a high quality score are more likely to be euploid (6), and euploid embryo transfers have a better chance of implanting and growing into a healthy baby.
Preimplantation Genetic Testing (PGT-A)
In addition to embryo grading, embryologists can conduct PGT-A on the embryos, which tests a few cells from a blastocyst-stage embryo to ensure that they are euploid. In the vast majority of cases, the test doesn’t harm the embryo. At the blastocyst stage, the embryo has split into two types of cells – the cells that will form the fetus (inner cell mass) and the cells that will form the placenta (trophectoderm) – and the embryologist only removes cells from the trophectoderm layer. PGT-A testing can increase your chance of a live birth per transfer by ensuring that only euploid blastocysts are transferred to your uterus.
At first glance, PGT-A appears to be the better test. However, the usefulness of PGT-A is still up for debate. Some downsides to PGT-A include:
-
It’s expensive. Though some clinics include the cost of PGT-A in the cost of IVF, it can cost hundreds to thousands of dollars per cycle to test the embryos.
-
PGT-A can result in false positive and negative results, meaning that it can occasionally identify a euploid embryo as aneuploid, and vice versa. One possible reason for this is that the test takes only a few cells from the embryo’s outer layer of cells, and those cells may not fully represent the ratio of aneuploid vs. euploid cells in the embryo.
-
PGT-A can result in the discard of normal embryos or damage embryos. A study conducted by Alife data scientists estimated that while there was a 0% loss for younger patients, patients of advanced maternal age experienced a 3-16% loss of viable embryos.
-
PGT-A may not improve outcomes for good-prognosis patients under 37. One study found that for patients under 37 with 3 or more good quality blastocyst-stage embryos, conducting PGT-A on the embryos did not improve the probability of a live birth (7).
It’s important to note that PGT-A testing will not improve your overall rate of success with IVF, it will only improve your success rate per embryo transfer. In other words, PGT-A testing can’t improve the embryos that result from your IVF cycle, it can only shorten your journey through IVF by helping to identify euploid embryos that may have a better chance of implantation, pregnancy, and a healthy baby.
Recent Articles
References
-
Mikwar, Myy, et al. “Mechanisms of Oocyte Aneuploidy Associated with Advanced Maternal Age.” Mutation Research/Reviews in Mutation Research, vol. 785, July 2020, p. 108320, https://doi.org/10.1016/j.mrrev.2020.108320.
-
Capalbo, Antonio, et al. “On the Reproductive Capabilities of Aneuploid Human Preimplantation Embryos.” The American Journal of Human Genetics, vol. 109, no. 9, Sept. 2022, pp. 1572–1581, https://doi.org/10.1016/j.ajhg.2022.07.009. Accessed 9 Feb. 2023.
-
Barbash-Hazan, Shiri, et al. “Preimplantation Aneuploid Embryos Undergo Self-Correction in Correlation with Their Developmental Potential.” Fertility and Sterility, vol. 92, no. 3, 1 Sept. 2009, pp. 890–896, pubmed.ncbi.nlm.nih.gov/18829021/, https://doi.org/10.1016/j.fertnstert.2008.07.1761. Accessed 7 Apr. 2023.
-
Viotti, Manuel, et al. “Using Outcome Data from One Thousand Mosaic Embryo Transfers to Formulate an Embryo Ranking System for Clinical Use.” Fertility and Sterility, vol. 115, no. 5, May 2021, pp. 1212–1224, https://doi.org/10.1016/j.fertnstert.2020.11.041. Accessed 23 July 2022.
-
Capalbo, Antonio, et al. “Mosaic Human Preimplantation Embryos and Their Developmental Potential in a Prospective, Non-Selection Clinical Trial.” The American Journal of Human Genetics, vol. 108, no. 12, Dec. 2021, pp. 2238–2247, https://doi.org/10.1016/j.ajhg.2021.11.002.
-
Alfarawati, Samer, et al. “The Relationship between Blastocyst Morphology, Chromosomal Abnormality, and Embryo Gender.” Fertility and Sterility, vol. 95, no. 2, Feb. 2011, pp. 520–524, https://doi.org/10.1016/j.fertnstert.2010.04.003.
-
Yan, Junhao, et al. “Live Birth with or without Preimplantation Genetic Testing for Aneuploidy.” New England Journal of Medicine, vol. 385, no. 22, 25 Nov. 2021, pp. 2047–2058, https://doi.org/10.1056/nejmoa2103613.
Images:
Demko, Zachary P., et al. “Effects of Maternal Age on Euploidy Rates in a Large Cohort of Embryos Analyzed with 24-Chromosome Single-Nucleotide Polymorphism–Based Preimplantation Genetic Screening.” Fertility and Sterility, vol. 105, no. 5, May 2016, pp. 1307–1313, www.sciencedirect.com/science/article/pii/S0015028216000662, https://doi.org/10.1016/j.fertnstert.2016.01.025.
Viotti, Manuel, et al. “Using Outcome Data from One Thousand Mosaic Embryo Transfers to Formulate an Embryo Ranking System for Clinical Use.” Fertility and Sterility, vol. 115, no. 5, May 2021, pp. 1212–1224, https://doi.org/10.1016/j.fertnstert.2020.11.041. Accessed 23 July 2022.
Share this
Recent Articles
Learn everything you need to know about IVF
Join the newsletter for IVF education, updates on new research, and early access to Alife products.



