What Is PGT Testing and How Does It Work?
Medically reviewed by Linda Streety, RN, BSN
Preimplantation Genetic Testing (PGT) is a test that screens for chromosomal or genetic abnormalities in an embryo created through in vitro fertilization (IVF). Once your eggs have been retrieved and fertilized, an embryologist takes a small sample of cells from the outer layer of an embryo to analyze. This test increases the likelihood that you will transfer chromosomally normal embryos, which give you the best chance of success.
You will get your PGT results about 1-2 weeks after the sample has been sent for testing. Because it takes some time to conduct the test, embryos that undergo PGT will need to be frozen (cryopreserved) for transfer at a later time. Once your PGT results arrive, you and your care team can select an embryo for transfer.
Keep in mind, PGT is a diagnostic test and cannot change embryos. It won’t improve your overall live birth rate (your chance of having a baby with IVF), but it will improve the likelihood that your embryo transfer will be successful and result in a healthy baby.
Do I have to do PGT?
PGT is an add-on procedure that you can choose to do during IVF, and it is not mandatory by any means. If you opt not to do PGT, your embryos will still be assessed for quality in a process called embryo grading. All embryos created through IVF that reach a certain stage of growth are graded by an embryologist, who assesses the morphology (size and shape) of the embryo. This helps the embryologist prioritize certain embryos for transfer. Embryos with better grades are more likely to implant and lead to a successful pregnancy. For good-prognosis patients under 37 years old, PGT may not improve the cumulative live birth rate (1) and embryo grading may be an effective assessment.
You may choose to do PGT if you want to ensure that you are only transferring euploid (chromosomally normal) embryos, or if you are concerned about certain genetic conditions.
Types of PGT testing
Most patients who undergo PGT will do PGT-A, which screens for chromosomal abnormalities. However, there are different types of PGT, depending on your concerns. Below, we’ll describe the differences:
PGT-A
PGT-A testing screens embryos to make sure they have the correct number of chromosomes. More or fewer than the correct number of chromosomes can lead to a failure to implant, miscarriage, or a child with genetic conditions.
PGT-M
PGT-M screens for monogenic (single gene) disorders that could significantly alter quality of life (2), such as cystic fibrosis, sickle cell anemia, muscular dystrophy, or the BRCA1 or BRCA2 mutation that increases the chance of developing breast or ovarian cancer. PGT-M can check for these disorders if you or your partner have a family history of a monogenic disorder, are both carriers of an autosomal recessive disorder, or are both carriers of an autosomal dominant disorder.
PGT-P
This is a relatively new screening test for polygenic (caused by multiple genes) disorders (3) such as diabetes, cancer, and coronary artery disease. However, the use of PGT-P is controversial. Polygenic disorders are complex, with a strong environmental component, and PGT-P may not accurately screen for this complexity (4) . There are also concerns about how ethical PGT-P is, since it can be used to select desired traits as well as reduce disease risk (5).
PGT-SR
This test checks for structural abnormalities in an embryo’s chromosomes (6). If you or your partner has a chromosome rearrangement (i.e. balanced translocation), PGT-SR can check for this in the embryo. These abnormalities can increase your chance of miscarriage (7).
How accurate is PGT?
Because PGT takes only a small sample of cells from an embryo, there’s a chance that the sample is not representative of what is happening in the embryo. This is particularly the case with mosaic embryos, which have a mix of genetically normal (euploid) and abnormal (aneuploid) cells. In the case of mosaic embryos, the embryo can sometimes self-correct and result in a healthy baby, especially if a lower percentage of the cells are aneuploid (8). Because of this – even though mosaic embryos have a lower chance of success (9) – you may choose to retain mosaic embryos for potential transfer.
Also, while PGT raises the chance that an embryo will implant and lead to a live birth, it is not a guarantee.
Drawbacks and risks of PGT
PGT can be expensive
It’s hard to give an exact cost since it greatly depends on the clinic and the number of embryos being biopsied, tested, and frozen. Total costs can be as low as $2,000, but can go up significantly with subsequent rounds of IVF. Because PGT-A is rarely covered by insurance, for many patients, the procedure can become a significant percentage of the overall cost of IVF.
Occasional false positives or negatives
Because PGT only tests a small sample of cells from the embryo, it’s possible to get a false positive or negative result. In fact, some clinicians think PGT-A shouldn’t be used as a diagnostic tool because healthy babies have been born from embryos identified as aneuploid (10).
Potential damage to embryo
In the vast majority of cases, PGT does not affect the embryo. This is because at the blastocyst stage of development, the embryo has separated into two types of cells: the outer layer (trophectoderm, or TE) that will develop the placenta and other pregnancy-supporting membranes, and the inner cell mass (ICM) which will become the fetus. PGT only removes cells from the TE and does not take any cells that might develop into a fetus. Still, there is always a small risk of damage. Alife’s data researchers estimated that for patients under 36, PGT-A resulted in no loss of embryos. However, patients over 36 may have experienced a loss of 3-16% with PGT-A. This research was done at a single fertility center and more research will need to be done to understand the loss of embryos through PGT.
Loss from multiple thaw cycles
You may choose to do PGT on fresh embryos that are then frozen. You may also choose to thaw already frozen embryos to conduct PGT, and freeze them again. However, repeated freeze-thaw cycles can result in a lower chance of live birth (11).
Benefits of PGT
Improved implantation success for some patients
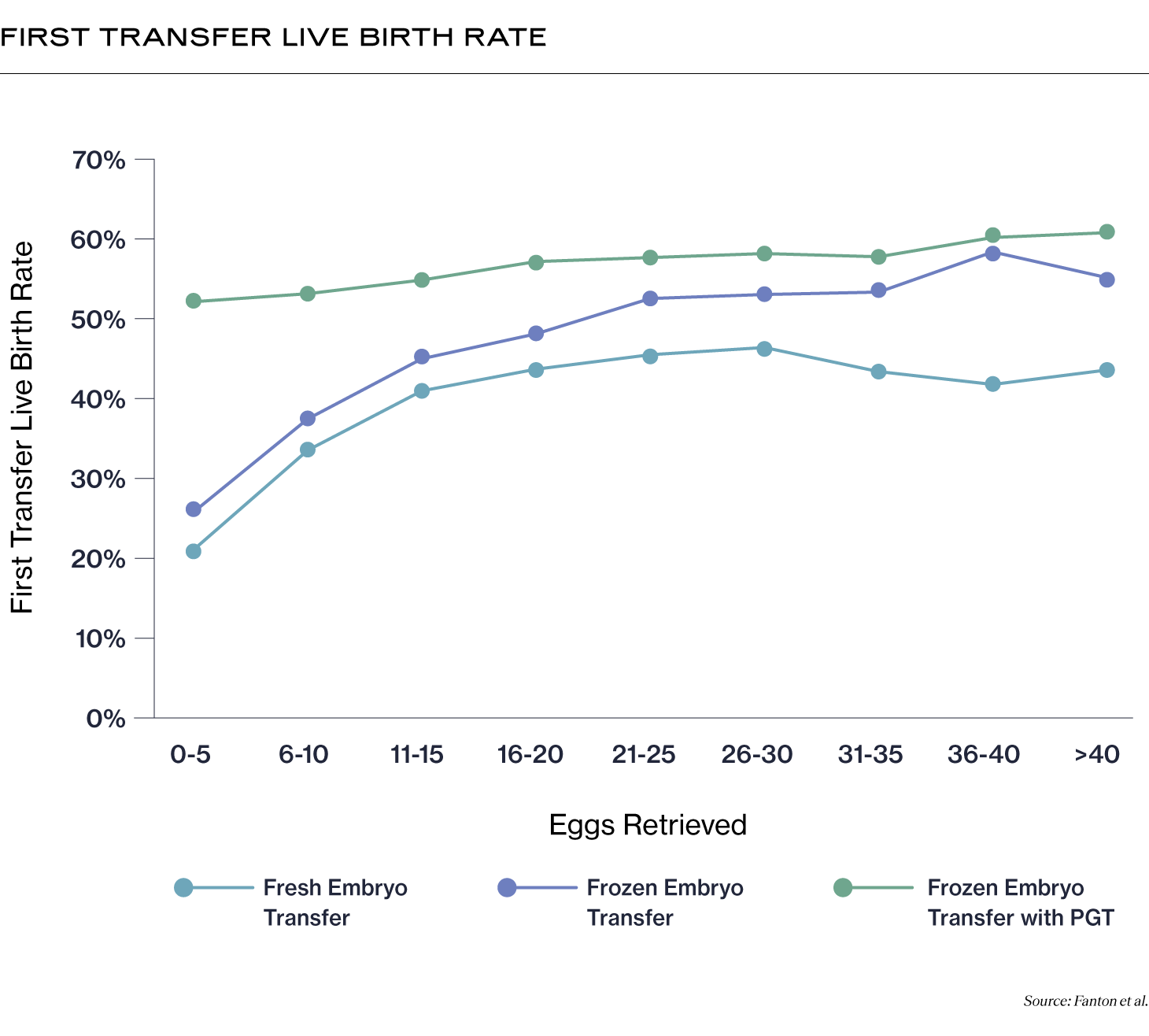
For patients who have experienced recurrent miscarriages or repeated implantation failure, PGT-A may help. One study showed that PGT-A may increase your chance of a live birth per transfer to about the same as a patient without those concerns (12). PGT-A may also help improve success rates per transfer for older patients (13). For patients older than 36, the live birth rate per transfer improved to 54.1% from 32.8%. You can also see, in the graphic below, how live birth rates for first transfer improve for patients who undergo PGT (14). (Please note that one potential reason why fresh transfer rates are lower may be because of elevated estradiol levels during fresh transfers, and because fresh transfers may be recommended for low prognosis patients.)
Reduced time undergoing IVF
While PGT can’t improve your overall chance of success with IVF, it can shorten the amount of time that you are undergoing IVF by helping to identify which embryos are more likely to lead to a successful transfer and live birth (15).
Peace of mind
PGT can greatly reduce anxiety if you have serious concerns about genetic disorders or chromosomal abnormalities.
Deciding to do PGT is a highly personal decision. If you’re interested in PGT, it’s important to talk to your fertility care team before starting your IVF cycle to weigh your options and determine whether PGT is the right choice for you.
Recent Articles
References
-
Yan, J., Qin, Y., Zhao, H., Sun, Y., Gong, F., Li, R., Sun, X., Ling, X., Li, H., Hao, C., Tan, J., Yang, J., Zhu, Y., Liu, F., Chen, D., Wei, D., Lu, J., Ni, T., Zhou, W., & Wu, K. (2021a). Live Birth with or without Preimplantation Genetic Testing for Aneuploidy. New England Journal of Medicine, 385(22), 2047–2058. https://doi.org/10.1056/nejmoa2103613
-
De Rycke, M., & Berckmoes, V. (2020). Preimplantation Genetic Testing for Monogenic Disorders. Genes, 11(8), 871. https://doi.org/10.3390/genes11080871
-
Treff, N. R., Eccles, J., Marin, D., Messick, E., Lello, L., Gerber, J., Xu, J., & Tellier, L. C. A. M. (2020). Preimplantation Genetic Testing for Polygenic Disease Relative Risk Reduction: Evaluation of Genomic Index Performance in 11,883 Adult Sibling Pairs. Genes, 11(6), 648. https://doi.org/10.3390/genes11060648
-
Forzano, F., Antonova, O., Clarke, A., de Wert, G., Hentze, S., Jamshidi, Y., Moreau, Y., Perola, M., Prokopenko, I., Read, A., Reymond, A., Stefansdottir, V., van El, C., & Genuardi, M. (2022). The use of polygenic risk scores in pre-implantation genetic testing: an unproven, unethical practice. European Journal of Human Genetics, 30(5), 493–495. https://doi.org/10.1038/s41431-021-01000-x
-
Treff, N. R., Eccles, J., Lello, L., Bechor, E., Hsu, J., Plunkett, K., Zimmerman, R., Rana, B., Samoilenko, A., Hsu, S., & Tellier, L. C. A. M. (2019). Utility and First Clinical Application of Screening Embryos for Polygenic Disease Risk Reduction. Frontiers in Endocrinology, 10. https://doi.org/10.3389/fendo.2019.00845
-
Scriven, P. N. (2021). PGT-SR (reciprocal translocation) using trophectoderm sampling and next-generation sequencing: insights from a virtual trial. Journal of Assisted Reproduction and Genetics. https://doi.org/10.1007/s10815-021-02174-5
-
Morin, S. J., Eccles, J., Iturriaga, A., & Zimmerman, R. S. (2017). Translocations, inversions and other chromosome rearrangements. Fertility and Sterility, 107(1), 19–26. https://doi.org/10.1016/j.fertnstert.2016.10.013
-
Barbash-Hazan, S., Frumkin, T., Malcov, M., Yaron, Y., Cohen, T., Azem, F., Amit, A., & Ben-Yosef, D. (2009a). Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertility and Sterility, 92(3), 890–896. https://doi.org/10.1016/j.fertnstert.2008.07.1761
-
Viotti, M., Victor, A. R., Barnes, F. L., Zouves, C. G., Besser, A. G., Grifo, J. A., Cheng, E.-H., Lee, M.-S., Horcajadas, J. A., Corti, L., Fiorentino, F., Spinella, F., Minasi, M. G., Greco, E., & Munné, S. (2021a). Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertility and Sterility, 115(5), 1212–1224. https://doi.org/10.1016/j.fertnstert.2020.11.041
-
Barad, D. H. (2022). Preimplantation genetic testing for aneuploidies screening is not diagnostic. F&S Reports. https://doi.org/10.1016/j.xfre.2022.11.004
-
Aluko, A., Vaughan, D. A., Modest, A. M., Penzias, A. S., Hacker, M. R., Thornton, K., & Sakkas, D. (2021). Multiple cryopreservation–warming cycles, coupled with blastocyst biopsy, negatively affect IVF outcomes. Reproductive BioMedicine Online, 42(3), 572–578. https://doi.org/10.1016/j.rbmo.2020.11.019
-
Lee, Chun-I., Wu, C.-H., Pai, Y.-P., Chang, Y.-J., Chen, Chung-I., Lee, T.-H., & Lee, M.-S. (2019). Performance of preimplantation genetic testing for aneuploidy in IVF cycles for patients with advanced maternal age, repeat implantation failure, and idiopathic recurrent miscarriage. Taiwanese Journal of Obstetrics and Gynecology, 58(2), 239–243. https://doi.org/10.1016/j.tjog.2019.01.013
-
Murphy, L. A., Seidler, E. A., Vaughan, D. A., Resetkova, N., Penzias, A. S., Toth, T. L., Thornton, K. L., & Sakkas, D. (2019). To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A). Human Reproduction (Oxford, England), 34(2), 268–275. https://doi.org/10.1093/humrep/dey346
-
Fanton, M., Cho, J. H., Baker, V. L., & Loewke, K. (2023a). A higher number of oocytes retrieved is associated with an increase in 2PNs, blastocysts, and cumulative live birth rates. Fertility and Sterility. https://doi.org/10.1016/j.fertnstert.2023.01.001
-
Neal, S. A., Morin, S. J., Franasiak, J. M., Goodman, L. R., Juneau, C. R., Forman, E. J., Werner, M. D., & Scott, R. T. (2018a). Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertility and Sterility, 110(5), 896–904. https://doi.org/10.1016/j.fertnstert.2018.06.021
Share this
Recent Articles
Learn everything you need to know about IVF
Join the newsletter for IVF education, updates on new research, and early access to Alife products.



