Embryo Development: From Egg to Implantation
Medically reviewed by Linda Streety, RN, BSN
The basics of conception are pretty simple: an egg and sperm combine to create an embryo which implants in the uterus, grows into a fetus, and becomes a baby! But if you’re undergoing in vitro fertilization (IVF), you will likely become familiar with many more of the details of how this happens. Below, we’ll talk about how eggs grow, what has to happen for successful fertilization, and how the resulting embryo implants in the uterine wall.
It may help to start with some of the more technical terms you’ll encounter:
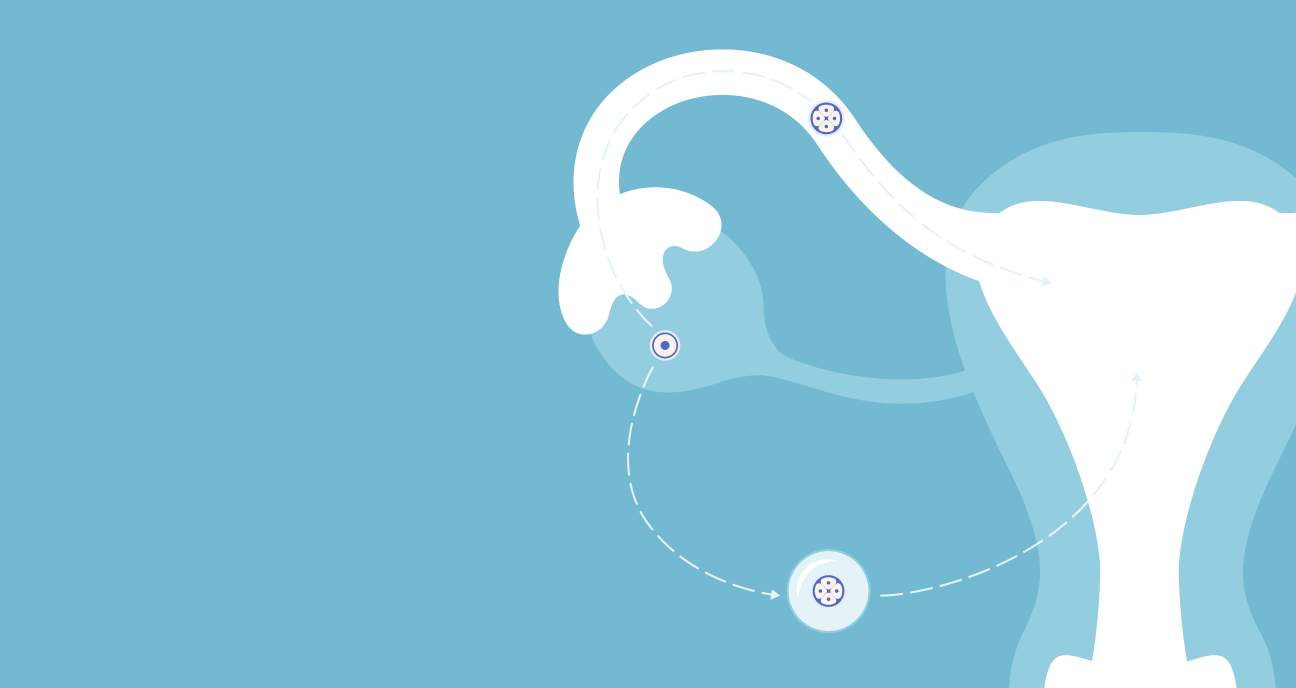
What is a follicle?
Follicles are tiny sacs inside the ovaries. Each follicle contains an immature egg. The vast majority of these “primordial” stage follicles will stay dormant, die by attrition, or begin developing but degenerate and be reabsorbed in a process called “atresia.” However, some of these follicles will grow and mature – along with the egg inside them – and eventually ovulate the egg.
What is a blastocyst stage embryo?
About 5 days after fertilization, the resulting embryo reaches the “blastocyst” stage. At this point, the embryo has separated into two types of cells, the inner cell mass (ICM) and trophectoderm (TE). The ICM will eventually form the baby, and the TE will help the embryo implant in the uterine wall and form the placenta. Many fertilized eggs will not develop to the blastocyst stage (scroll to the bottom of this article to see the percentage of fertilized eggs that reach the blastocyst stage by patient's age).
What is a euploid embryo?
Also known as a “normal” embryo, a euploid embryo has 23 pairs of chromosomes. Euploid embryos are more likely to implant, less likely to result in miscarriage, and less likely to result in a baby with intellectual or physical challenges caused by chromosomal abnormalities.
With that knowledge, it’s easier to understand the process from follicle to implantation. Every embryo that becomes a fetus undergoes these steps, whether the pregnancy is unassisted or assisted. However, the procedures required to help the embryo through the steps are more involved in an assisted pregnancy. Below, we’ll go through each step.
1. Egg Growth and Maturation
The process of waking up primordial follicles in the ovaries and developing them is controlled by two hormones from your pituitary gland – follicle stimulating hormone (FSH) and luteinizing hormone (LH). It takes roughly a year for a primordial follicle to develop a mature egg. And those follicles are not alone - a group of about 10-20 follicles reach the final stage of development together.
Unassisted Reproduction
Most of this group of 10-20 follicles die by atresia, a process by which eggs stop developing, break down, and are reabsorbed by the body. However, one dominant follicle (the biggest one) will reach maturity about midway through one of your menstrual cycles.
As your follicles grow, levels of estrogen also rise in your blood. At a certain point, this rise in estrogen triggers a surge of LH which causes the dominant follicle to mature. This means that the egg inside the follicle will shed half of its chromosomes (from 46 to 23) in a process known as “meiosis.” After the egg has matured, it will be “ovulated,” or released into your fallopian tube for its chance to be fertilized.
Assisted Reproduction
Your reproductive endocrinologist (RE) will use hormone medications to stimulate your ovaries (also known as “ovarian stimulation”) to develop more of this group of about 10-20 follicles to maturity while preventing premature ovulation (so you don’t ovulate before the RE can retrieve your eggs).
Once the dominant follicle(s) has reached about 18-20 mm in size, your RE will schedule the “trigger shot,” which will encourage your follicles to mature and undergo meiosis. Then your RE carefully schedules the egg retrieval procedure to occur approximately 34-36 hours after the trigger shot so they can capture the eggs before they are ovulated. During the egg retrieval procedure, you will be lightly sedated while the RE uses an ultrasound to guide a thin needle through your vaginal wall and into each of your ovaries to retrieve your eggs.
2. Fertilization
Once a sperm cell has entered an egg cell, the egg undergoes rapid changes. The zona pellucida (the membrane “shell” surrounding the egg) immediately becomes impervious to any other sperm. The sperm and egg chromosomes combine to form 23 pairs of chromosomes that provide the roadmap for a growing fetus.
Unassisted Reproduction
After ovulation, the egg slowly travels down the fallopian tube for about 12-24 hours. There may already be sperm in the fallopian tube, waiting for the egg (sperm can live up to 5 days in the female reproductive tract!) Or sperm may reach the fallopian tubes during the egg’s progress inside the tube.
Assisted Reproduction
After egg retrieval, the embryologist will carefully check each egg to ensure that it is mature. Then the embryologist will combine the eggs with sperm in a petri dish and allow them to fertilize. If the fertility clinicians have identified issues with sperm size and shape (morphology) or ability to swim (motility), they may choose a more intensive method of fertilization known as intracytoplasmic sperm injection (ICSI). Fertilization with ICSI involves injecting individual sperm into individual eggs. When the embryologist sees two pronuclei inside the cell – one from the sperm and one from the egg – they know that fertilization has been successful.
3. Embryo Growth to Blastocyst Stage Embryo
A fertilized egg becomes a single-cell embryo known as a “zygote.” This cell rapidly divides in a process known as “mitosis.” By day 3, embryos that have reached the zygote stage have about 8 cells. But by day 5-7, the cells of embryos that have reached the “blastocyst” stage have differentiated into two types. The ball of cells forming the inner cell mass (ICM) will eventually become the fetus, and an outer layer of cells known as the trophectoderm (TCM) will become the placenta and other supporting structures for the fetus.
Unassisted Reproduction
The developing embryo descends rapidly into the uterus, where the uterine lining has been thickening for the past two weeks in preparation for its arrival.
Assisted Reproduction
The embryo can be transferred fresh on day 3 or day 5 of development, meaning that it is transferred 3-5 days after egg retrieval. It can also be frozen at day 3 or day 5 of development for later implantation.
Before transferring an embryo, the embryologist carefully assesses, or “grades,” the form and structure of the embryo. Embryos with higher grades have a better chance of implanting and developing into a healthy baby. The embryologist can also perform preimplantation genetic testing (PGT) on blastocyst stage embryos to test for chromosomal or genetic abnormalities so you only transfer euploid embryos. PGT involves taking a few cells from the trophectoderm layer of the embryo. Embryos that have undergone PGT must be frozen for later transfer.
When your body is ready for embryo transfer, your RE will load the embryo into a slim catheter and use an ultrasound to guide it through your cervix and into your uterus, where it will be released.
4. Implantation
The embryo hatches out of its protective shell (the zona pellucida) and continues to rapidly grow. About 1-3 days after hatching, the trophectoderm cells adhere to and burrow into the uterine lining (sometimes referred to as the “endometrial” lining), enabling the growth of the vascular structure that will support the pregnancy. The hormone human chorionic gonadotropin (hCG) enters the parent’s bloodstream, and rapidly builds until it’s detectable in a beta hCG blood test.
Unassisted Reproduction
The follicle that released the egg from the ovary becomes a structure known as the corpus luteum, which produces progesterone. This hormone supports early pregnancy.
Assisted Reproduction
If you are not producing enough progesterone, your RE may prescribe progesterone supplementation in the weeks before and after embryo transfer. They may also prescribe estrogen or GnRH supplementation to help build your uterine lining before embryo transfer to give you the best chance of success.
How many eggs does a person with ovaries have?
If you have ovaries, this will change throughout your life. While you are born with 1-2 million microscopic follicles in your ovaries, this number decreases to 300,000-400,000 follicles by puberty. Your ovarian reserve continues to decline throughout your life, even though you may only ovulate 300-400 eggs. Below is a graph that shows the average number of follicles in your ovaries from birth to menopause (1).
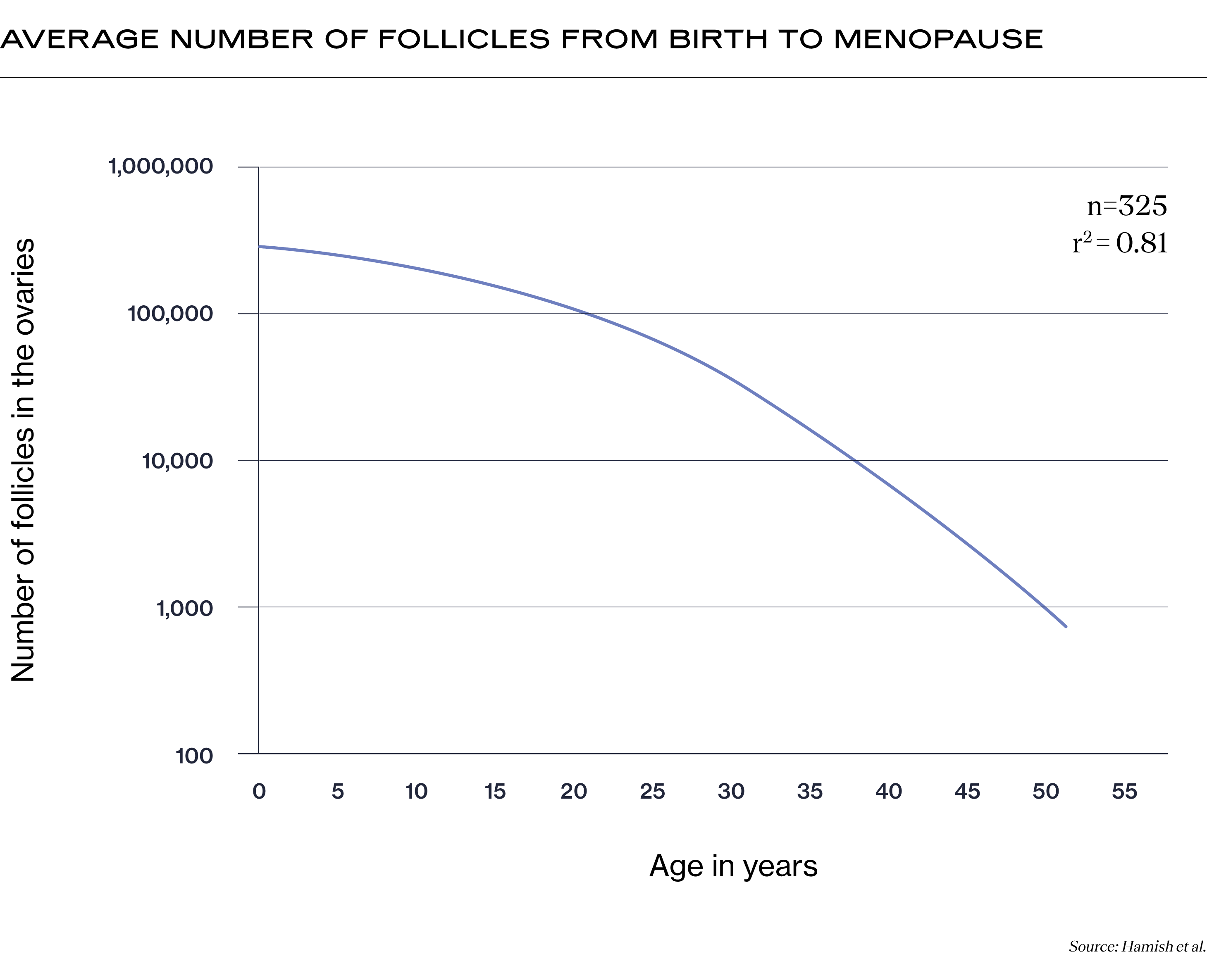
Interestingly, scientists and clinicians long believed that if you had ovaries, you were born with all the follicles (and therefore all the eggs) you would ever grow. However, new research challenges this assumption. Stem cells in the ovaries may have the potential to continue to develop new eggs throughout a person’s life (2). While much more research on this topic is still needed, the hope is that exploring this may lead to developments that could help extend a person’s reproductive timeline.
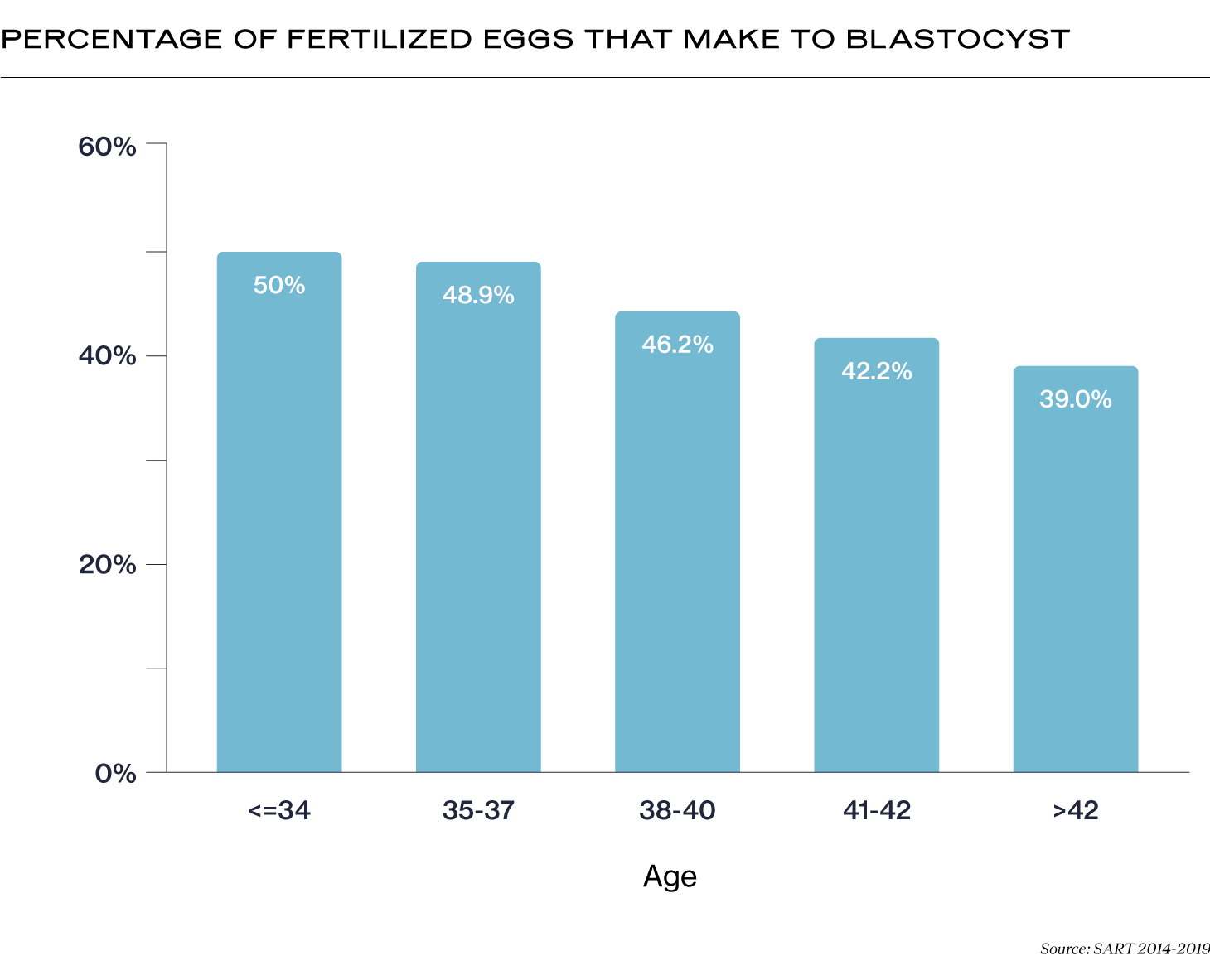
What percentage of fertilized eggs make it to blastocyst?
The percentage of fertilized eggs that make it to the blastocyst stage during IVF decreases with maternal age at the time of egg retrieval. According to data collected by the Society for Assisted Reproductive Technology (SART) between 2014-2019, while the percentage of fertilized eggs that make it to blastocyst is about 50% for patients under 34, this percentage decreases to 39% for patients over 42 years old. Please note that these percentages are for the age of the patient at the time of egg retrieval; age of the patient at the time of fertilization and embryo transfer are less important.
Recent Articles
References
-
Wallace, W. H. B., & Kelsey, T. W. (2010). Human Ovarian Reserve from Conception to the Menopause. PLoS ONE, 5(1). https://doi.org/10.1371/journal.pone.0008772
-
Virant-Klun, I. (2015). Postnatal oogenesis in humans: a review of recent findings. Stem Cells and Cloning: Advances and Applications, 49. https://doi.org/10.2147/sccaa.s32650
Share this
Recent Articles
Learn everything you need to know about IVF
Join the newsletter for IVF education, updates on new research, and early access to Alife products.



